News & Notes > Screening Procedures on bioRxiv
Screening Procedures on bioRxiv
bioRxiv | 2022-06-13
To increase the transparency of bioRxiv’s manuscript screening process, we describe here the requirements for submission and details of how submitted manuscripts are dealt with. We hope this information, together with our site FAQs, will help authors prepare manuscripts that can be handled with minimal delay.
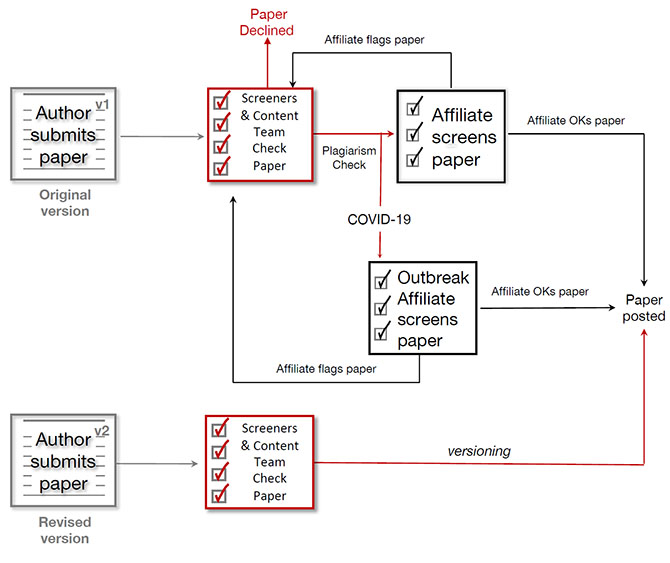
Overview of the screening process
Manuscripts submitted to bioRxiv undergo two screening steps. First, in-house screening staff with scientific and editorial expertise check that the details inputted during submission are complete - e.g. the authors have included the appropriate information in the submission fields, special characters are coded correctly, and any group authors are not incorrectly listed as individual authors. The in-house screeners also ensure the manuscript itself is within scope (see below) and an appropriate article type: a research manuscript is acceptable; a narrative review, commentary, opinion piece, or step-by-step protocol is not. bioRxiv is intended for disseminating research articles with new data. All article types, such as protocols and bioinformatic tools must be written in the context of a research article and must contain data. Results can include use-case examples, outputs or reanalysis of previously published data. In addition, they check for the presence of overt identifying information, such as photographs of patients or study participants.Submissions also undergo automated text analyses to identify potential plagiarism and/or material that has already appeared online (e.g. in a journal or on another preprint server).
The second screening stage is the responsibility of volunteer Principal Investigators known as bioRxiv Affiliates. Affiliates consider two main questions: Does the manuscript present biological research? And is there potential for public harm from posting it as a preprint? If Affiliates conclude the submission is not biological research or they have concerns about the content, the submission is flagged for further discussion in-house.
Manuscript escalation
Manuscripts can be escalated at any stage in the screening process for discussion by the Content Team and, where needed, by the bioRxiv Founders and individual Affiliates or external advisors. Submissions can be escalated for a number of reasons, for example:- If the article type or content is not within scope (such as public health research),
- If the manuscript contains conclusions that may cause harm or scare to the public (e.g. data challenging known toxicity/carcinogenicity, such as disputing tobacco-cancer connections).
These discussions may result in the return of the manuscript to the authors to address the concerns that have been raised. A submission may also be declined as better disseminated after peer review, most often because of the potential negative impact of the findings on the public. Approximately 5% of bioRxiv submissions are found not to meet our criteria for posting.
The screening process typically takes 24–48 hours, but can take longer over weekends and holidays, or if the submission requires in-house discussion and further correspondence with authors. Authors should note that manuscripts cannot be posted at a specific date or time, or their release embargoed according to a requested schedule.
